[ad_1]

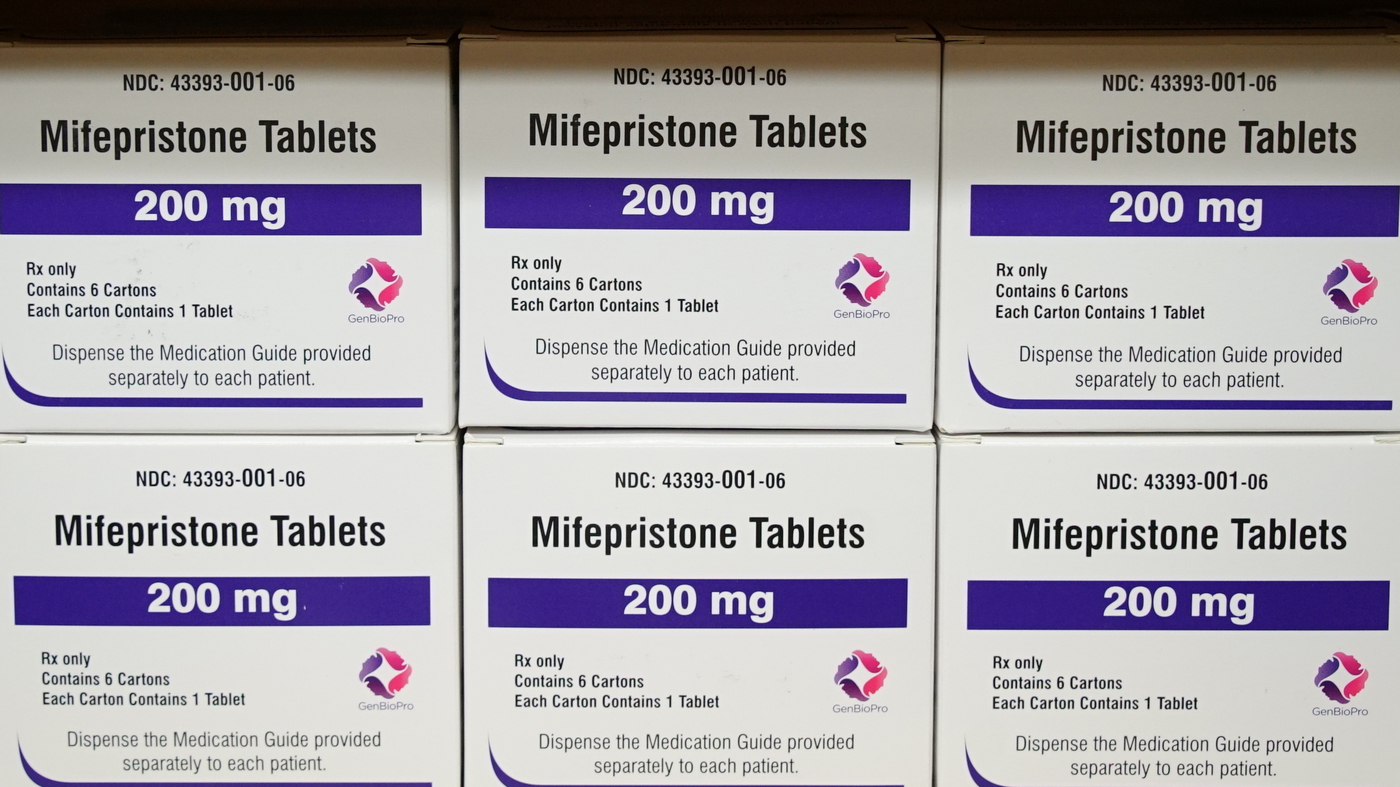
Mifepristone is portion of a two-drug protocol that a recent review confirmed was used in 98% of medication abortions in 2020.
Allen G. Breed/AP
disguise caption
toggle caption
Allen G. Breed/AP
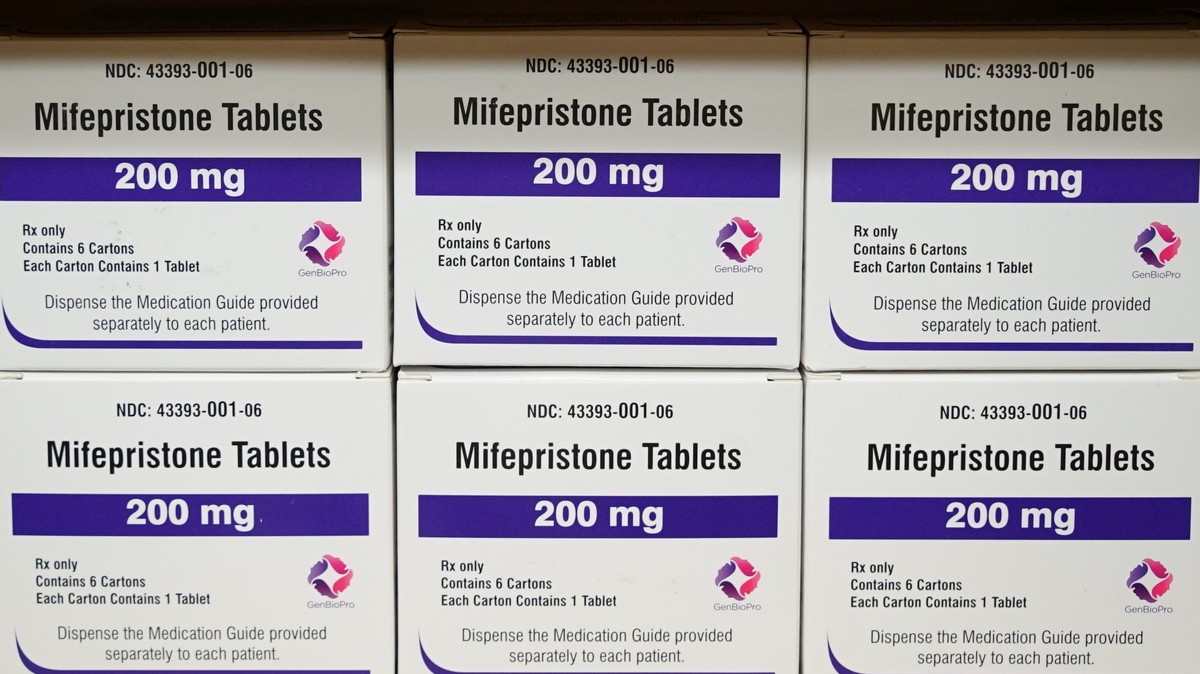
Mifepristone is aspect of a two-drug protocol that a modern examine showed was employed in 98% of medicine abortions in 2020.
Allen G. Breed/AP
Federal judges in two states issued contradictory decisions Friday evening that could substantially influence accessibility to a drug applied in almost all treatment abortions in the U.S.
In Texas, U.S. District Choose Matthew Kacsmaryk ruled that the Foodstuff and Drug Administration improperly approved the abortion pill mifepristone a lot more than 20 years ago. A coalition of anti-abortion legal rights groups called the Alliance for Hippocratic Medicine sued the Fda past 12 months. The judge issued a nationwide injunction pausing the FDA’s acceptance, which is set to consider result in 7 times.
Within just several hours of that choice, U.S. District Judge Thomas O. Rice issued a ruling in a different scenario in Washington condition. That lawsuit filed by a coalition of Democratic lawyers typical in 17 states and the District of Columbia sought to block the Food and drug administration from pulling the drug from the market.
Rice’s selection blocks the Food and drug administration from “altering the position quo and rights as it relates to the availability of Mifepristone.”
Washington condition Lawyer Common Bob Ferguson advised NPR on Friday that he thinks the judge’s ruling could make it attainable for sufferers in those states to keep on making use of mifepristone for abortion in the short term — even after the Texas determination takes effect.
It is really not obvious how each individual judge’s decision will affect the other, and the two scenarios are probably to conclude up just before the U.S. Supreme Court.
Hrs following the Texas ruling, the Justice Office appealed to the U.S. Courtroom of Appeals for the Fifth Circuit, which has a standing for staying a conservative jurisdiction. The Justice Office suggests it is also reviewing the decision in Washington point out.
President Biden stated the ruling in Texas could have prevalent penalties. “If this ruling have been to stand, then there will be almost no prescription, accepted by the Fda, that would be safe and sound from these sorts of political, ideological assaults,” the president explained in a statement.
“It is the up coming massive step toward the nationwide ban on abortion that Republican elected officers have vowed to make legislation in The usa,” Biden added.
Anti-abortion legal rights teams hailed the Texas determination. “By illegally approving perilous chemical abortion medication, the Fda put women of all ages and women in harm’s way, and it is really higher time the agency is held accountable for its reckless steps,” Erik Baptist, senior counsel with Alliance Defending Independence, reported in a assertion.
Mifepristone was authorized by the Food and drug administration in 2000 for use in blend with a second drug, misoprostol. Far more than 50 % of all abortions in the United States are finished utilizing medication, as opposed to a surgical treatment, and the two-drug blend was employed for 98% of them in 2020, in accordance to the Guttmacher Institute.
In its lawsuit, the coalition of abortion legal rights opponents mentioned the protocol was improperly permitted by the Fda. The team experienced questioned Kacsmaryk, who was appointed by President Trump and has longstanding ties to conservative religious teams, to overturn the approval.
The selection in that lawsuit comes a few months after Kacsmaryk held a listening to in Amarillo in a courtroom that experienced space for only a number of dozen customers of the public and the push. No recording or general public livestreaming was permitted.
Nationwide implications
Abortion suppliers nationwide say they’ve been planning to count on a different treatment abortion regimen making use of misoprostol by yourself. Misoprostol is recommended mostly for ulcers, and is previously commonly utilised off-label for other gynecological functions in the United States.
Investigate indicates the single-drug program is considerably significantly less helpful and generally triggers further aspect results. But the Entire world Overall health Firm says the process, which has been employed internationally for a long time, can be risk-free and helpful at the appropriate dosage.
The determination very likely will necessarily mean uncertainty and confusion for medical professionals and clients, states Farah Diaz-Tello, senior counsel with the reproductive legal rights authorized advocacy group If/When/How.
“People today who are trying to find an abortion with capsules … are heading to find it considerably additional tricky to do so, specifically in the time time period as vendors determine out what they are heading to be ready to do,” she claims. “So I imagine we’re going to see an speedy exacerbation of the crisis of entry that already started out in June of 2022” with the U.S. Supreme Court docket selection last calendar year in Dobbs v. Jackson Women’s Wellness Corporation, which overturned a long time of abortion-legal rights precedent.
Diaz-Tello predicts far more men and women will glance to induce their possess abortions devoid of professional medical supervision, applying drugs received on-line or in other international locations. She also anxieties about the chance of improved scrutiny of people trying to get health-related care for emergency issues from possibly self-managed abortions or miscarriages.
She says there are no condition guidelines to her know-how that need health care companies to switch in clients suspected of inducing an abortion, but she problems the ruling will fuel confusion and misinformation.
“I am fearful that … that is likely to translate into a misunderstanding that is likely to lead to the criminalization of people today who conclusion their pregnancies,” Diaz-Tello says.
Dueling decisions
The implications of the Texas ruling is intricate by the result of the Washington point out lawsuit.
Amanda Allen, senior counsel and director for the The Lawyering Project, which supports abortion legal rights, says the two courts “could occur out with two pretty conflicting orders, and they could impose really distinctive obligations on the Fda that would be very untenable for the Food and drug administration to check out to reconcile.”
Allen says the Fda could make your mind up to problem guidance for prescribers about how to interpret the rulings. But she suggests these kinds of a conflict between the federal courts may well perfectly end up in advance of the U.S. Supreme Courtroom.
[ad_2]
Source website link