[ad_1]
Why It Issues: Some people today continue to be at hazard.
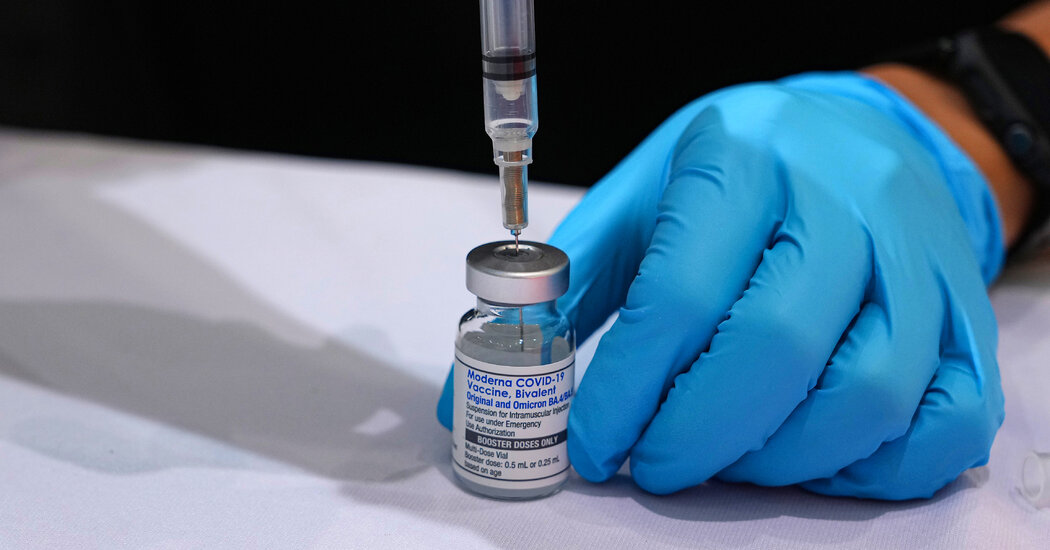
Pfizer, Moderna and Novavax have designed it clear that they have to have time to make tens of tens of millions of doses of the photographs that would be out there in the slide.
“I think which is what today’s dialogue is about — how to best to appear up with what goes into people’s arms to provide the greatest protection during a period when we assume we’ll have waning immunity,” reported Dr. Peter Marks, the F.D.A.’s vaccine chief. He included that the winter season may possibly also deliver “further evolution of the virus.”
Given that the starting of the pandemic, 6.2 million hospitalizations and 1.1 million deaths have been attributed to the virus in the United States, according to details introduced by Natalie Thornburg, a vaccine skilled at the Facilities for Disorder Control and Prevention.
She mentioned the photo had improved this 12 months, but people who continue being vulnerable involve the unvaccinated, folks who are immunocompromised and these who have diabetic issues or serious kidney, lung, cardiovascular or neurologic ailments. People today 65 and more mature are also at danger, and that rises with age.
Qualifications: Modifications are afoot in who will get the photographs and when.
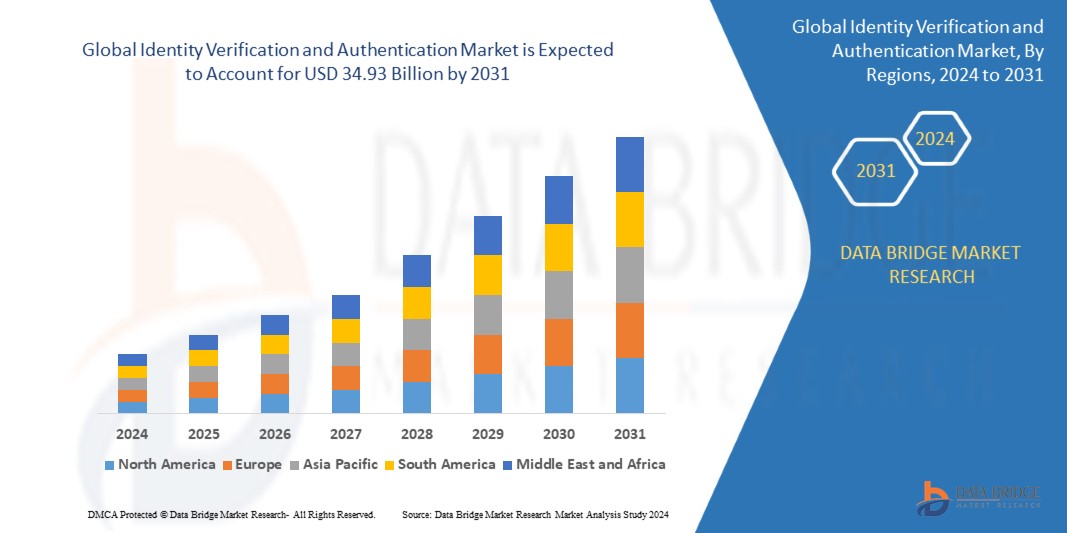
The bivalent shots made available previous slide provided protection towards the Omicron variant and an early Covid variant. About 20 p.c of grown ups, or about 53 million, in the United States acquired the booster shot, with the rates higher among the older adults.
Moving forward with a shot qualified at only an XBB variant usually means that newborns and folks with compromised immune units may not have immunity versus the earliest coronavirus variants. That need to not be a issue, in accordance to a briefing provided by a Entire world Well being Organization formal, who reported these variants have been no more time in circulation.
What’s Upcoming: A vaccine could be presented alongside flu and R.S.V. jabs.
The F.D.A. is anticipated to make a extra formal recommendation to vaccine makers soon. The producers will be predicted to analyze the new formulation and post information to the agency. If approvals are granted, the C.D.C. will advise overall health companies on which age teams need to get the jab.
An F.D.A. spokesman mentioned it envisioned that an current vaccine would be offered by late September, assuming the facts help protected and effective vaccines.
It stays unclear irrespective of whether or when the vaccine makers or the F.D.A. will look at the potential consequences of administering numerous vaccines in the drop, including people for the flu and respiratory syncytial virus, or R.S.V., which are envisioned to be readily available for expecting individuals and older older people. Company advisers have also endorsed the use of an R.S.V. antibody shot to guard infants.
[ad_2]
Supply link